K+ sink uncovered: How tumors trap potassium to evade immunity
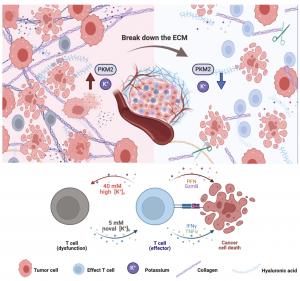
GA, UNITED STATES, June 25, 2025 /EINPresswire.com/ -- Understanding how tumors manipulate their microenvironment is key to improving cancer immunotherapy. A recent study has revealed that PKM2, a metabolic enzyme released from necrotic tumor cells, acts as a “potassium sink” within tumor interstitial fluid (TIF). This phenomenon traps potassium ions (K+), creating an immunosuppressive environment that dampens CD8+ T cell activity and promotes tumor growth. The research demonstrated that PKM2 binds hundreds of potassium ions through electrostatic interactions, with enhanced binding under the acidic (pH 6.5) conditions typical of tumors. These findings provide critical insights into ionic regulation within tumors and highlight potential strategies—such as collagenase-mediated enzymatic remodeling of the tumor stroma—to restore immune function and enhance therapeutic outcomes.
The tumor microenvironment (TME) includes not only cancer cells and stroma but also the tumor interstitial fluid (TIF), which plays a pivotal role in shaping immune responses. Potassium ion accumulation in TIF has been linked to suppression of T cell function, yet the mechanism of K+ retention remained unclear. While earlier studies focused on cellular interactions and immune checkpoints, less attention has been paid to biophysical factors such as ion gradients. Potassium imbalance within tumors has emerged as a potent inhibitor of immune cell activation. These challenges motivated further research to understand how tumors retain potassium and exploit it to escape immune surveillance.
Researchers from the Institute of Biophysics, Chinese Academy of Sciences, etc., published their findings (DOI: 10.1093/procel/pwae036) on June 24, 2024, in Protein & Cell, detailing how the glycolytic enzyme PKM2 regulates potassium ion retention in TIF. Using patient-derived samples and mouse tumor models, the team found that extracellular PKM2 traps potassium ions, promoting immune evasion. This discovery sheds light on how necrotic tumor cells shape ionic dynamics and suggests that manipulating the tumor matrix could alleviate immune suppression. The work bridges molecular metabolism and immune microenvironment, providing new therapeutic targets.
This letter-style study investigated how potassium ions accumulate in TIF, a hallmark of immunosuppressive tumors. Analysis of tissue from non-small cell lung cancer patients and several mouse tumor models confirmed elevated K+ concentration in TIF, while sodium, calcium, and chloride ions remained unchanged. Proteomic profiling revealed that TIF is rich in cytoplasmic proteins, notably PKM2, released from necrotic cells. PKM2 exists primarily in a tetrameric form (240 kDa) and is highly abundant in tumors. Using equilibrium dialysis and protein structural analysis, researchers demonstrated that PKM2 binds 300–400 potassium ions per molecule via electrostatic interactions, driven by pH-dependent conformational changes. Furthermore, collagenase digestion of the tumor stroma significantly reduced TIF K+ levels and PKM2 abundance, restoring CD8+ T cell function and inhibiting tumor growth specifically in immune-competent mice (not immunodeficient models). Mutational analysis of PKM2’s ion-binding sites confirmed the necessity of structural conformation for K+ binding capacity. The study proposes that the “K+ sink” formed by extracellular PKM2 contributes to immune evasion and that targeting this mechanism may enhance the efficacy of cancer immunotherapy.
This study offers a compelling molecular explanation for how tumors retain potassium and suppress immune responses, said Dr. Wenfeng Zeng, one of the co-corresponding authors. By identifying PKM2 as a key potassium-binding protein in the tumor stroma, the researchers now have a potential target to reverse immune suppression. Their findings suggest that disrupting the TME’s ionic balance—e.g., via collagenase—could become a viable strategy to enhance current immunotherapy protocols.
The discovery of PKM2 as a potassium ion sink opens new avenues for therapeutic intervention. By disrupting potassium retention in the TIF—through enzymatic matrix degradation or targeting PKM2 directly—it may be possible to reinvigorate immune responses within tumors. These strategies could complement existing checkpoint inhibitors or adoptive T cell therapies. Moreover, monitoring PKM2 levels or ionic profiles in tumors may serve as a diagnostic tool to assess immunosuppressive status. As the field moves toward integrated tumor profiling, ionic regulation may become a critical component in designing personalized cancer treatments.
DOI
10.1093/procel/pwae036
Original Source URL
https://doi.org/10.1093/procel/pwae036
Funding information
This work was supported by the National Natural Science Foundation of China (81702823 to W.Z.), the Priority Research Program from the Chinese Academy of Sciences (XDB37010100 to P.Z.), and the Basic Research Program Based on Major Scientific Infrastructures of Chinese Academy of Sciences (JZHKYPT-2021-05 to P.Z.). The experimental protocols of this study were approved by the Animal Care and Use Committee of the Institute of Biophysics, Chinese Academy of Science, China (SYXK2021128), The use of tissues from patients with NSCLC was approved by the ethics committees at the Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China (NCC2022C-702, from 8 June 2022), and all experiments were performed in accordance with relevant guidelines and regulations.
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.