Cancer Diagnostics Market Forecast 2026–2036: Revenue to Reach USD 165.8 Billion at 8.8% CAGR
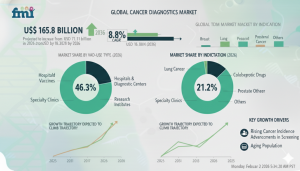
DE, UNITED STATES, February 20, 2026 /EINPresswire.com/ -- The global Cancer Diagnostics Market is projected to generate USD 71.1 billion in 2026 and expand to USD 165.8 billion by 2036, registering a robust CAGR of 8.8%. Expansion is being driven by rising precision oncology adoption, broader integration of molecular assays, and stronger emphasis on early and accurate cancer detection across hospitals and specialized laboratories.
Higher testing volumes, increased availability of next-generation sequencing platforms, and expanding use of liquid biopsy technologies are reshaping oncology diagnostics. As biomarker-driven therapy selection becomes routine, diagnostic systems are evolving beyond conventional tissue-based approaches toward integrated genomic, proteomic, and metabolomic platforms.
Market Snapshot
Industry Value (2026): USD 71.1 Billion
Industry Forecast Value (2036): USD 165.8 Billion
CAGR (2026–2036): 8.8%
Leading End User: Hospitals & Diagnostic Centers (46.3%)
High-Growth Regions: Asia Pacific, North America, Europe
Key Companies: Roche Diagnostics, Abbott Diagnostics, Thermo Fisher Scientific Inc., Illumina Inc., Guardant Health Inc.
What Is Driving Precision Oncology Adoption?
The shift toward tumor biology-based treatment decisions is significantly accelerating demand for companion diagnostics and biomarker-focused platforms. Tumor mutation burden testing, pharmacogenomic analysis, and mutation tracking are supporting more targeted immunotherapy and chemotherapy strategies.
Companion diagnostics linked to newly launched targeted therapies are expanding routine testing requirements. At the same time, ongoing mutation monitoring for resistance and response assessment is increasing repeat testing volumes, strengthening revenue stability for advanced diagnostic platforms.
Segment Insights
Hospitals & Diagnostic Centers Lead End-User Demand (46.3%)
Hospitals and diagnostic centers are projected to hold 46.3% of market share in 2026 due to concentrated infrastructure, specialist staffing, and accredited laboratory systems. Their integrated oncology workflows support diagnosis, treatment planning, and follow-up monitoring within regulated environments.
Automation investments and high-throughput molecular systems are further strengthening their role as primary testing hubs for precision oncology services.
Breast Cancer Remains the Largest Clinical Focus (21.2%)
Breast cancer diagnostics are expected to account for 21.2% of market share in 2026, supported by high screening intensity and standardized biomarker testing protocols. Routine hormone receptor and HER2 assessments guide therapy selection, while genomic profiling adoption continues expanding in specialized oncology settings.
Liquid Biopsy Emerges as Key Innovation Area (28.4%)
Liquid biopsy is projected to represent 28.4% of test-type share in 2026. Minimally invasive blood-based testing supports repeat sampling and improved patient access, complementing tissue-based diagnostics.
Advances in circulating tumor DNA (ctDNA) sensitivity and circulating tumor cell detection are reinforcing adoption across multiple cancer indications, particularly in minimal residual disease monitoring and treatment response tracking.
How AI and Precision Platforms Are Reshaping Revenue Models
Growing integration of artificial intelligence into digital pathology systems is increasing demand for platforms capable of automated image analysis, case triage, and decision support. Laboratories are prioritizing scalable systems that combine scanning, storage, and AI-enabled interpretation to improve throughput and consistency.
Precision medicine integration is also reshaping test distribution. Molecular tumor boards and protocol-based ordering systems are encouraging structured, repeatable test usage rather than isolated single-order diagnostics. Companion diagnostics tied to specific therapies are reinforcing sustained testing demand throughout treatment cycles.
Regulatory Evolution and Market Impact
Stricter validation and clinical utility standards are increasing development requirements but strengthening provider confidence in advanced assays. Companion diagnostic regulations are reinforcing linkage between test performance and therapeutic labeling claims, improving long-term adoption stability across major markets.
Country-Level Growth Outlook (2026–2036)
India (7.2%) is emerging as a high-growth market driven by expanded screening initiatives, infrastructure upgrades, and growing medical travel demand.
China (6.9%) benefits from precision medicine policy support and genomic research investments.
The United States (5.8%) continues to expand through companion diagnostic partnerships and advanced testing integration.
Japan (5.3%) is supported by an aging population and established screening programs.
Germany (4.7%) remains a quality-focused market emphasizing personalized oncology pathways and laboratory modernization.
Competitive Landscape
Competition in the cancer diagnostics market centers on advanced molecular capabilities, biomarker validation strength, and oncology-specific platform integration.
Major participants including key companies shaping competitive dynamics include:
Foundation Medicine
Exact Sciences Corporation
Qiagen N.V.
Agilent Technologies
Bio-Rad Laboratories
Innovation priorities include next-generation sequencing expansion, high-sensitivity molecular testing, digital pathology integration, and workflow automation. While consolidation remains steady, companies are increasingly acquiring biotech assets to secure proprietary biomarkers and strengthen development pipelines.
Get Access of Report Sample:https://www.futuremarketinsights.com/reports/sample/rep-gb-1090
Market Segmentation Overview
By Test Type
Tumor Biomarker Tests
Biopsy
Liquid Biopsy
Immunohistochemistry
In Situ Hybridization
Others
By Indication
Breast Cancer
Lung Cancer
Colorectal Cancer
Prostate Cancer
Blood Cancer
Others
By End User
Hospitals & Diagnostic Centers
Independent Laboratories
Cancer Research Institutes
Others
By Biomarker Type
Genetic Biomarkers
Protein Biomarkers
Metabolic Biomarkers
Others
Frequently Asked Questions
What will be the value of the cancer diagnostics market by 2036?
The market is projected to reach USD 165.8 billion.
What is the expected CAGR from 2026 to 2036?
The industry is forecast to grow at an 8.8% CAGR.
Which end-user segment leads the market?
Hospitals & diagnostic centers lead with 46.3% share in 2026.
Which cancer type accounts for the largest share?
Breast cancer represents 21.2% of the market in 2026.
Explore More Related Studies Published by FMI Research:
Lung Cancer Diagnostics Market:https://www.futuremarketinsights.com/reports/lung-cancer-diagnostics-market
Liver Cancer Diagnostics Market:https://www.futuremarketinsights.com/reports/liver-cancer-diagnostics-market
Brain Cancer Diagnostics Market:https://www.futuremarketinsights.com/reports/brain-cancer-diagnostics-market
Breast Cancer Diagnostics Market:https://www.futuremarketinsights.com/reports/breast-cancer-diagnostics-market
Thyroid Cancer Diagnostics Market:https://www.futuremarketinsights.com/reports/thyroid-cancer-diagnostics-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Why FMI: Decisions that Change Outcomes- https://www.futuremarketinsights.com/why-fmi
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
Sudip Saha
Future Market Insights Inc.
+ +1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.